Heart Rhythm Congress 22
Posted on: in Blog
Exciting to think that Stereax batteries may one day save someone’s life. My legal department reminds me that these batteries are not yet certified for in-body applications, but sometime soon they will be. One day a person will benefit from carrying an implanted cardiac monitoring device powered by our miniature batteries. With this thought in mind, I recently attended Heart Rhythm Congress 22 in Birmingham, UK, aiming to learn more about the industry that manufactures pacemakers, cardiac sensing monitors, defibrillators etc.
HRC22 offered plenty of oral presentations summarizing studies and trials taking place all over the world but particularly in the UK - from Glasgow, Middlesborough, Liverpool, Oxford or London to name a few. Outside the lecture rooms though, in the exhibition hall, the biggest medical device companies were not UK-based, but mostly from the USA or Europe.
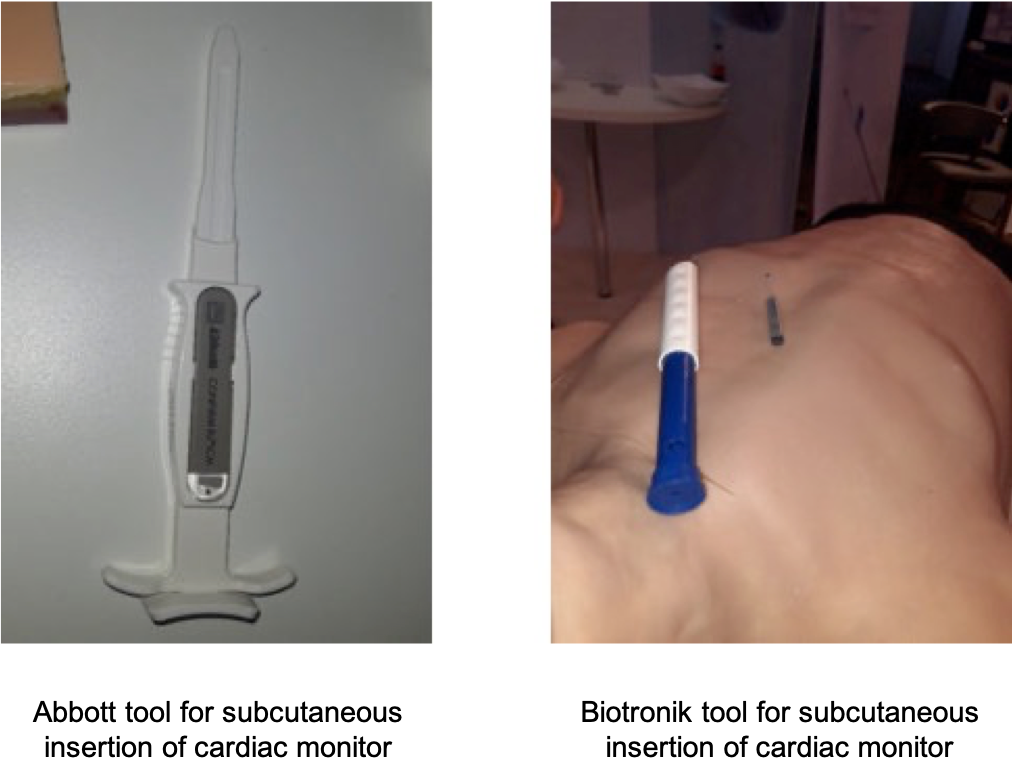
For example, I saw small cardiac sensors that could be inserted subcutaneously into the chest and remain there for several years, regularly measuring ECG information and sending data wirelessly to the patient’s phone using BLE or proprietary communications protocols. With my Stereax product manager’s hat on, these devices attracted my attention since they tend to use very small batteries because the cardiac monitors themselves are usually less than 1 cm wide and about 1-2 mm thick. I saw such devices displayed by Abbott and Biotronik at HRC22. For the uninitiated, all devices appeared to be very similar, but in reality, each company aims to differentiate themselves by providing either the longest battery life, being the smallest in the market, or being the most accurate in the market (by being a little bit longer, electrodes are further apart, and a better quality information is measured).
Implanting a pacemaker in the chest and connecting leads through the chambers of the heart is a complicated and risky surgical procedure, especially for the older patient who may be having a replacement pacemaker as the battery in their old one has stopped working. Most pacemakers are large, mainly because of the battery which dominates the volume of the implants. It has to be large because it has several functionalities, including defibrillation in case of heart attack, keeping heart rate above a particular bpm, and various of types of sensing. And it must last many years. However with the unstoppable trend towards miniaturization, companies are looking to produce smaller implantable devices. These not only require less space in the body but can also be easier to insert resulting in a less risky and invasive implantation procedure. Microport displayed the ENO, one of the smallest in the market, which differs itself through SonR, a technology that continually measures the contractility of the heart.

Sometimes, insertion of traditional pacemaker leads to the heart is not possible and so smaller, leadless pacemakers are required despite not providing the full functionality of traditional pacemakers. Medtronic showed the catheter used for implanting their Micra leadless pacemaker. It has an outside diameter of 27 French (8.91 mm) and internal diameter of 23 French (7.59 mm). No surprise these companies are in need of very small and narrow batteries! Another manufacturer, Abbott, expects that their Aveir leadless will be introduced to the European market next year with a 10 year lifetime.

“Can a mm-size battery help” I asked some of the exhibitors. Yes, definitely, but the answer is not so simple. Patient’s low compliance towards regular battery recharging and FDA auditing indicate that only non-life critical applications could be certified which are powered by a small rechargeable battery. Perhaps in the near future though, a miniature battery, complemented by kinetic energy-harvesting technology (such as that developed by Cairdac who harvest the energy of the beating heart), cardiac devices could become even smaller, longer life and save more lives.
